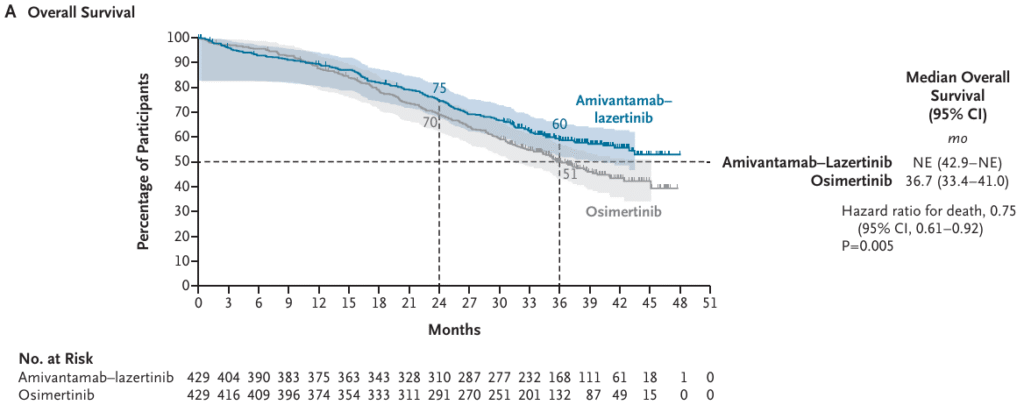
In September 2025, the overall survival results of the MARIPOSA trial were published in the NEJM. The phase 3 MARIPOSA trial evaluated amivantamab–lazertinib versus osimertinib in patients with previously untreated EGFR-mutated advanced non–small-cell lung cancer (NSCLC). With a median follow-up of nearly 37.8 months, the combination demonstrated a significant overall survival advantage compared with osimertinib alone. Three-year overall survival was 60% with amivantamab–lazertinib versus 51% with osimertinib, translating into a 25% reduction in the risk of death (HR 0.75, 95% CI 0.61–0.92; P=0.005)
Beyond overall survival, the combination delayed symptomatic progression (median 43.6 vs. 29.3 months) and extended the time to treatment discontinuation and need for subsequent therapy. Notably, outcomes were favorable across most subgroups, including patients with baseline brain metastases. Intracranial control was sustained, with 36% of patients on the combination remaining progression-free at 36 months versus 18% on osimertinib .
The safety profile of amivantamab–lazertinib was consistent with known effects of EGFR and MET inhibition, with higher rates of grade ≥3 adverse events (80% vs. 52%), including rash, infusion reactions, and venous thromboembolism. Importantly, no new safety signals emerged, and strategies such as dermatologic prophylaxis, anticoagulation, and the development of subcutaneous formulations may help improve tolerability. Taken together, MARIPOSA establishes amivantamab–lazertinib as an option for first-line EGFR-mutated advanced NSCLC, offering a meaning survival benefit over single agent osimertinib.
Although the MARIPOSA trial represents a major advance in the treatment landscape for EGFR-mutated NSCLC, the optimal sequencing of available therapies remains an evolving question. At WCLC 2025, the FLAURA2 trial reported a significant overall survival benefit with the combination of osimertinib plus cisplatin/carboplatin and pemetrexed compared to osimertinib alone in the first-line setting. As treatment options continue to expand, decisions must be individualized at the patient level, taking into account clinical factors such as tolerability, the treating team’s familiarity with new agents, and the financial feasibility of new costly therapies.